Conductive Ink Success
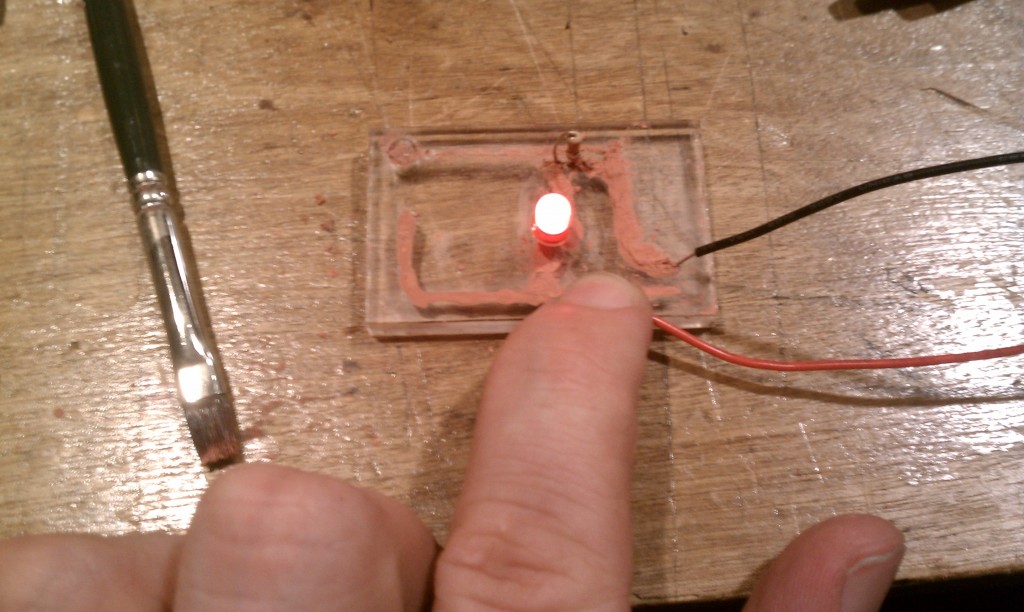
I’ve recently had success in making a conductive ink using a fine copper powder suspended in an acrylic airbrush medium. This paper on conductive epoxies was really the key to getting this ink working.
The paper shows that etching the metal filler slightly before mixing it with the binder improves the conductivity of the ink. In this test I first used ammonium persulfate as the initial etching solution. After decanting off the resulting copper sulfate solution, the powder was then washed with deionized water. The wet powder was then mixed with an acrylic airbrush medium to make the resulting ink.
There is still quite a bit of experimentation to be done, but this is a very encouraging result!
If you are interested in conductive inks you should look at some of these projects:
- This ink made at Pumping station one that results in bulk silver
- Kit-of-No-Parts Amazing projects
- And of course openMaterials
I have been told that the problem with using copper powder in conductive inks is that copper oxide does not conduct well. As your ink oxidizes, the sheet resistivity of the cured ink will rise over time. If you can get the copper to sinter together to form a solid network, this problem can be resolved, but this is tricky and requires high heat. This is the reason that silver powder is used for low resistance conductive inks in industry… silver oxide conducts very well, so the sheet resistivity changes much less over time.
Good luck with the project!
I’d treat it in a galvanic bath afterwards to put a tiny but solid copper surface on it.
Nice thing is, you’d only need a mediocre conductivity of the ink (plus you already have some coppers sulfate 😉
Tried this with selfmade conductive ink but my experiments with silver powder and graphite didn’t work out. I’ll test your method now!
Cheers,
Mathias
My hope is that copper oxides won’t form within the binder matrix. I have also added a small amount of a weak acid (citric) to the ink to inhibit the formation of oxides on the copper. A long term solution would be to electroplate the traces, which I have had success with on nickel based ink.
What about mixing in a little powdered indium? that ought to do the trick, and its not particularly expensive.
Indium tin oxide is also used as a transparent conductor and tin oxide has been used since the late 1940’s for front coatings on night vision tubes, Nixie pinball displays and other applications.
I even have a flat CRT here where the final anode is obviously a transparent conductor.
Try etching with citric acid rather than
ammonium persulfate as the initial etching solution.
I believe this would work better.
Where did you get the ammonium persulfate?
Hello! Could you please tell me why you chose the airbrush acrylic medium over,lets say, waterproof ink?